Expert Commentary

Why FDA hearing on morcellation safety could drive innovation
Reaction from Cheryl Iglesia, MD, Advisory Panel Member, to FDA’s 2-Day Hearing

Ceana Nezhat, MD, and Erica Dun, MD, of the Atlanta Center for Minimally Invasive Surgery and Reproductive Medicine, Atlanta, Georgia, present this surgical case of a 41-year-old G0 with radiating lower abdominal pain and mennorhagia who desired removal of her symptomatic myomas. Preoperative transvaginal ultrasound revealed a 4-cm posterior pedunculated myoma and 5-cm fundal intramural myoma.

Reaction from Cheryl Iglesia, MD, Advisory Panel Member, to FDA’s 2-Day Hearing
James D. Kondrup, MD, demonstrates his surgical team's approach to morcellation, with use of a morcellation bag after laparoscopic supracervical...
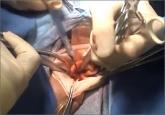
Ceana Nezhat, MD, and Erica Dun, MD, show how they perform enclosed vaginal morcellation
Approximately one in 368 women undergoing hysterectomy have undetected uterine cancer, according to a new study, which did not specify the types...
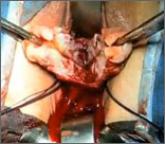
Dr. Shibley discusses a novel strategy he has developed to address the problem of tissue dispersion during open power morcellation
This technique is K. Anthony Shibley, MD's, short-term surgical solution to performing closed power morcellation. His patented pneumoperitoneum...
