Recently, the US Preventive Services Task Force (USPSTF) finalized 7 recommendations on 5 topics and posted draft recommendations on an additional 10 topics. It also implemented new procedures that include posting draft recommendations for public comment (see “A new review process for the USPSTF”). This article reviews the USPSTF activity in 2011, as well as cervical cancer screening recommendations issued earlier this year.
In response to the adverse publicity from the 2009 mammogram recommendations and the increased scrutiny brought on by the affordable care act—which mandates that A and B recommendations from the US Preventive Services Task force are covered preventive services provided at no charge to the patient—the USPSTF developed and implemented a new review procedure. This is intended to increase stakeholder involvement at all steps in the process.
Last year, the USPSTF completed its rollout of this new online review process. The USPSTF now posts all draft recommendations and the evidence report supporting them on its Web site for public comment. final recommendations are posted months later after consideration of the public input. The final recommendations for the 10 topics with draft recommendations posted in 2011 are expected to be released this year.
Potential for confusion. The new process may cause confusion for family physicians. Draft recommendations will receive press coverage and may differ from the final recommendations, as happened with cervical cancer screening recommendations. Physicians will need to familiarize themselves with the process and look for final recommendations on the USPSTF Web site at http://www.uspreventiveservicestaskforce.org/recommendations.htm.
2012 recommendations
Screening for cervical cancer
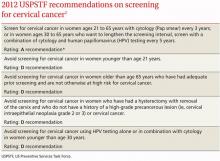
The USPSTF released its new recommendations on screening for cervical cancer in March (TABLE 1).1 The final document varied from the 2011 draft recommendations in 2 areas: the roles of human papillomavirus (HPV) testing and sexual history.
- The draft issued an I statement (insufficient evidence) for the role of HPV testing. Subsequently, based on stakeholder and public comment (as well as a review of 2 large recently published studies), the USPSTF gave an A recommendation to the use of HPV testing in conjunction with cervical cytology as an option for women ages 30 years and older who want to increase the interval between screening to 5 years.2,3
- The draft stated that the age at which screening should be initiated depends on a patient’s sexual history. The final recommendations state that screening should not begin until age 21, regardless of sexual history.
TABLE 1
*For more on the USPSTF's grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm.
These new recommendations balance the proven benefits of cervical cytology with the harms from overscreening and are now essentially the same as those of other organizations, including the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology. They differ in minor ways from those of the American Congress of Obstetricians and Gynecologists, and the American Academy of Family Physicians is assessing whether to endorse them.
Importantly, the new recommendations identify individuals for whom cervical cytology should be avoided—women younger than age 21, most women older than age 65, and those who have had a hysterectomy with removal of the cervix. A decision to stop screening after the 65th birthday depends on whether the patient has had adequate screening yielding normal findings: This is defined by the USPSTF as 3 consecutive negative cytology results (or 2 consecutive negative co-test results with cytology and HIV testing) within 10 years of the proposed time of cessation, with the most recent test having been performed within 5 years. Avoiding cytology testing after hysterectomy is contingent on the procedure having been performed for an indication other than a high-grade precancerous lesion or cervical cancer. In addition, the recommendations advise against HPV testing in women younger than age 30, as it offers little advantage and leads to much overdiagnosis.
Liquid vs conventional cytology. As a minor point, the USPSTF says the evidence clearly shows that liquid cytology offers no advantage over conventional cytology. But it recognizes that the screening method used is often not determined by the physician.
Recommendations finalized in 2011
TABLE 2 summarizes recommendations completed by the USPSTF last year.
Neonatal gonococcal eye infection prevention
The recommendation to use topical medication (erythromycin ointment) to prevent neonatal gonococcal eye infection is an update and reaffirmation of a previous recommendation. Blindness due to this disease has become rare in the United States because of the routine use of a neonatal topical antibiotic, and there is good evidence that it causes no significant harm. Its use continues to be recommended for all newborns.4