BY LAWRENCE F. EICHENFIELD, MD, and JENNA BOROK
Nevus sebaceous (NS) is considered a subtype of an epidermal nevus, a benign hamartoma that includes an excess or deficiency of structural elements of the skin, such as epidermis, sebaceous, and apocrine glands.1
Nevus sebaceous, also known as nevus sebaceous of Jadassohn, was first described in 1895 by Josef Jadassohn as a nevoid growth composed predominantly of sebaceous glands.2 Sebaceous glands are found everywhere on the body where hair is found and are located adjacent to hair follicles with ducts, through which sebum flows.3
NS clinically appears as a waxy, yellowish-orange to pink, hairless plaque, ranging from 1 cm to over 10 cm in size and usually located on the scalp, face, neck, or trunk.1 When the lesions are linear, they typically follow Blaschko lines.1 The lesions change over time. An infant’s lesion will be slightly raised and can be hardly noticeable. If the lesion is located on the scalp, it will remain hairless. During later childhood, the nevus may thicken. The lesions may become thicker and verrucous during adolescence.
Histologically, NS are benign hamartomas with epidermal, follicular, sebaceous, and apocrine elements.4 The malformation is primarily within the individual folliculosebaceous units.1 An infantile lesion will deviate little from normal skin as the follicular units are so small.1 During childhood, microscopically, it is easier to see misshapen follicles and thickened epidermis.1 During adolescence, the histological pattern is similar to that of an epidermal nevi, which includes acanthosis and fibroplasia of the papillary dermis.
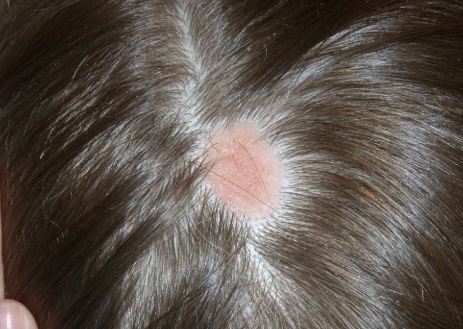
There are now several lines of evidence showing that nevus sebaceous is caused by genetic postzygotic mosaic mutations in the mitogen–activated protein kinase (MAPK) pathway, which specifically activate ras mutations including H ras and K ras genes.5-7 Since NS is caused by a somatic mosaicism mutation, there are a several syndromic findings associated with NS depending on the timing of the mutations during development and whether single versus multiple tissues are affected.8 Specifically, NS has been associated with Schimmelpenning-Feuerstein-Mims syndrome that includes NS, and skeletal, ocular, and neurologic abnormalities.1,7 A study found that cutaneous skeletal hypophosphatemia syndrome, manifested as NS and vitamin D–resistant rickets, has identical ras mutations in both skin and bone tissues, providing further support that these syndromic findings are a result of a multilineage somatic ras mutation.8
Diagnosis
NS is typically diagnosed clinically, based on the presence of a circumscribed, thin, yellow-orange, oval, round, or linear plaque, usually on the scalp or face. During infancy or the first stage, the lesions remain stable. In the second stage, during puberty, the lesions thicken and become verrucous or nodular because of changes in sebaceous gland activity that are driven by hormones. In the third stage of their natural course, benign and malignant epithelial neoplasms can develop, including trichoblastoma, syringocystadenoma papilliferum, sebaceous epithelioma, basal cell carcinoma, and trichilemmoma.9
The associated syndrome, nevus sebaceous syndrome, also known as Schimmelpenning syndrome, has more extensive cutaneous lesions along Blaschko lines and presents with CNS, ocular, or skeletal defects. The CNS abnormalities include mental retardation, seizures, and hemimegalencephaly.1 Therefore, a thorough neurologic and ophthalmologic examination should be performed in patients with suspected nevus sebaceous syndrome.
Aplasia cutis congenita is the absence of the skin at birth that presents with an erosion or deep ulceration to a scar or a defect that is covered with an epidermal membrane.1 Some aplasia cutis may present as a smooth, hairless surface at birth, making differentiation from nevus sebaceous difficult. The pinkish, orange to yellow hue of NS may help differentiate these entities. Juvenile xanthogranuloma is a fairly common histiocytosis and is the most common histiocytic disease of childhood.1 It is a benign proliferation of dermal dendrocytes, and it presents in infants as many red to yellow papules or a few nodules on the head and neck. Many lesions even remain undetected.1
Syringocystadenoma papilliferum is a benign neoplasm with apocrine differentiation and presents as a papule or plaque on the head and neck. It can be associated with nevus sebaceous.1
Treatment
The definitive treatment is an excision. The necessity and timing of excision of these lesions is still under debate.9 Secondary neoplasms do arise from the nevus sebaceous, although the incidence is low pre puberty.1 It has been estimated that 16% of cases develop benign tumors and that 8% of cases develop malignant tumors.9 The majority of malignant lesions are basal cell carcinomas, and a large recent study found that only 1% of patients had malignancies.10 While most of these tumors develop in adulthood, there have been reports of malignancies in children.10
An additional reason to excise NS is that, over time, they grow more verrucous, become inflamed, bleed with trauma, and may be unappealing cosmetically.1 Some experts recommend earlier excision in childhood, especially with larger lesions or facial lesions where minimizing deformity is important and with the possibility of less noticeable scarring.9 The prophylactic removal of NS remains controversial, and there is a lack of consensus among experts about the timing of excision. It is recommended that each lesion be evaluated on a case-by-case basis.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Borok is a medical student at the University of California, Los Angeles. Dr. Eichenfield and Ms. Borok said they had no relevant financial disclosures. Email them at pdnews@frontlinemedcom.com.
References
1. “Dermatology.” 3rd ed. (St Louis, Mo: Elsevier, 2012).
2. Arch Dermatol Res. 1895. doi: 10.1007/BF01842810.
3. J Invest Dermatol. 2004 Jul;123(1):1-12.
4. J Cutan Pathol. 1984;11(5):396-414.
5. J Invest Dermatol. 2013 Mar;133(3):827-30.
6. J Invest Dermatol. 2013 Mar;133(3):597-600.
7. Nat Genet. 2012 Jun 10;44(7):783-7.
8. JAAD. 2016 Aug;75(2):420-7.
9. Pediatr Dermatol. 2012 Jan-Feb;29(1):15-23.
10. Pediatr Dermatol. 2009 Nov-Dec;26(6):676-81.

