When selecting appropriate candidates for NTX treatment, clinicians should consider individuals who are:
- not interested in or able to receive agonist maintenance treatment (ie, patients who do not have access to an appropriate clinic in their area, or who are restricted to agonist treatment by probation/parole)
- highly abstinence-oriented (eg, active in a 12-step program)
- in professions where agonists are controversial (eg, healthcare and airlines)
- detoxified and abstinent but at risk for relapse.
Individuals who have failed agonist treatment (eg, who experience cravings for opioids and use opioids while receiving it, or are nonadherent or diverting/misusing the medication), who have a less severe form of OUD (short history and low level of use), or who use sporadically are also optimal candidates for NTX. Aside from the relapse-vulnerable washout gap prior to induction, one of the concerns with antagonist treatments is treatment retention; anecdotal clinical reports suggest that individuals often discontinue antagonists in favor of agonists.
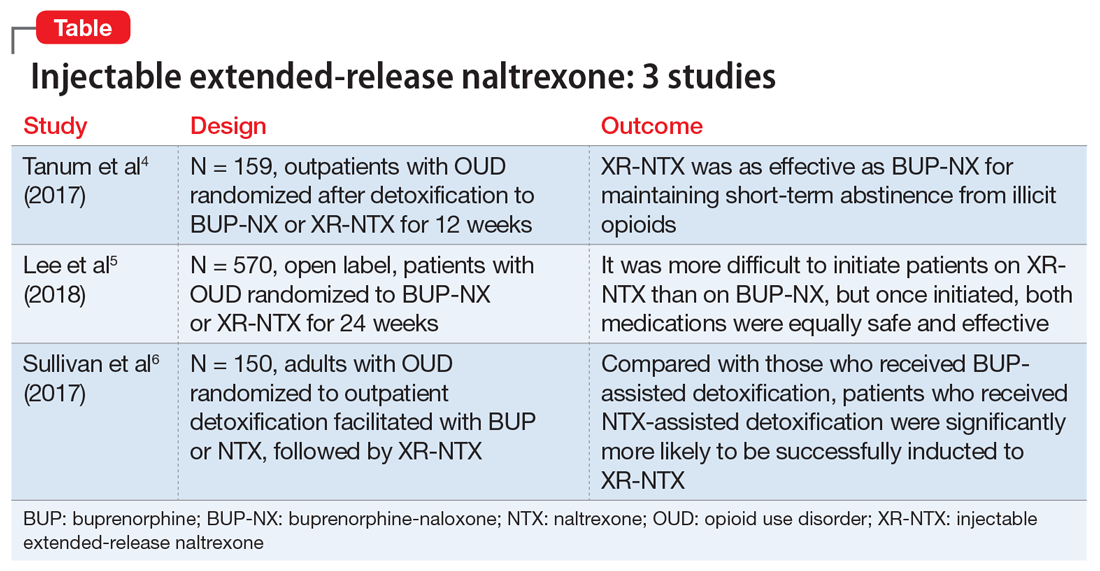
Several studies have investigated this by comparing XR-NTX with buprenorphine-naloxone (BUP-NX). Here we summarize 3 studies4-6 to describe which patients might be optimal candidates for XR-NTX, its success in comparison with BUP-NX, and challenges in induction of NTX, with a focus on emerging protocols (Table).
1. Tanum l, Solli KK, Latif ZH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197-1205.
This study aimed to determine whether XR-NTX was not inferior to BUP-NX in the treatment of OUD.
Study design
- N = 159, multicenter, randomized, 12-week outpatient study in Norway
- After detoxification, participants were randomized to receive BUP-NX, 4 to 24 mg/d, or XR-NTX, 380 mg/month.
Continue to: Outcomes