Psychiatry is experiencing a major paradigm shift.1 No longer is depression a disease of norepinephrine and serotonin deficiency. Today, we are exploring inflammation, methylation, epigenetics, and neuroplasticity as major players; we are using innovative treatment interventions such as ketamine, magnets, psilocin, anti-inflammatories, and even botulinum toxin.
In 2006, dermatologist Eric Finzi, MD, PhD, reported a case series of 10 depressed patients who were given a single course of botulinum toxin A (BTA, onabotulinum-toxinA) injections in the forehead.2 After 2 months, 9 out of the 10 patients were no longer depressed. The 10th patient, who reported improvement in symptoms but not remission, was the only patient with bipolar depression.
As a psychiatrist (M.M.) and a dermatologist (J.R.), we conducted a randomized controlled trial3 to challenge the difficult-to-swallow notion that a cosmetic intervention could help severely depressed patients. After reporting our positive findings and hearing numerous encouraging patient testimonials, we present a favorable review on the treatment of depression using BTA. We also present the top 10 questions we are asked at lectures about this novel treatment.
A deadly toxin used to treat medical conditions
Botulinum toxin is one of the deadliest substance known to man.4 It was named after the gram-positive bacterium Clostridium botulinum, which causes so-called floppy baby syndrome in infants who eat contaminated honey. Botulinum toxin prevents nerves from releasing acetylcholine, which causes muscle paralysis.
In the wrong hands, botulinum toxin can be exploited for chemical warfare.4 However, doctors are using it to treat >50 medical conditions, including migraine, cervical dystonia, strabismus, overactive bladder, urinary incontinence, excessive sweating, muscle spasm, and now depression.5,6 In 2014, BTA was the top cosmetic treatment in the United States, with >3 million procedures performed, generating more than 1 billion dollars in revenue.7
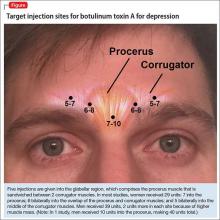
The most common site injected with BTA for cosmetic treatments is the glabellar region, which is the area directly above and in between the eyebrows (ie, the lower forehead). The glabella comprises 2 main muscles: the central procerus flanked by a pair of corrugators (Figure). When expressing fear, anger, sadness, or anguish, these muscles contract, causing the appearance of 2 vertical wrinkles, referred to as the “11s.” The wrinkles also can form the shape of an upside-down “U,” known as the omega sign.8 BTA prevents contraction of these muscles and therefore prevents the appearance of a furrowed brow. During cosmetic procedures, approximately 20 to 50 units of BTA are spread out over 5 glabellar injection sites.9 A similar technique is being used in studies of BTA for depression2,3,10,11 (Figure).
BTA for depression is new to the mental health world but, before psychiatrists caught on, dermatologists were aware that BTA could improve quality of life,12 reduce negative emotions,13 and increase feelings of well-being.14
The evidence
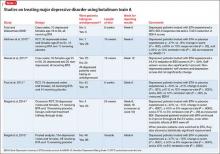
To date, there have been 2 case series,2,15 3 randomized control trials (RCTs),3,10,11 1 pooled analysis,16,17 and 1 meta-analysis18 looking at botulinum for depression (Table 1).2,10,11,15-17 In each trial, a single treatment of BTA (ie, 1 doctor’s visit; 29 to 40 units of BTA distributed into 5 glabellar injections sites), was the intervention studied.2
The first case series, by Finzi and Wasserman2 is described above. A second case series, published in 2013, describes 50 female patients, one-half depressed and one-half non-depressed, all of whom received 20 units of BTA into the glabella.15 At 12 weeks, depression scores in the depressed group had decreased by 54% (14.9 point drop on Beck Depression Inventory [BDI], P < .001) and self-esteem scores had increased significantly. In non-depressed participants, depression scores and self-esteem scores remained constant throughout the 12 weeks.
A pooled analysis reported results of 3 RCTs16,17 consisting of a total of 134 depressed patients, males and females age 18 to 65 who received BTA (n = 59) or placebo (n = 74) into the glabellar region. At the end of 6 weeks, BDI scores in the depressed group had decreased by 47.4% (14.3 points) compared with a 16.2% decrease (5.1 points) in the placebo group. This corresponds to a 52.5% vs 8.0% response rate and a 42.4% vs 8.0% remission rate, respectively (Table 1,1,2,10,11,15-17). There was no difference between the 2 groups in sex, age, depression severity, and number of antidepressants being taken. Females received 29 units and males received 10 to 11 units more to account for higher muscle mass (Figure).
Depression as measured by the physician-administered Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale showed similar reduction in overall scores (−45.7% vs −14.6%), response rates (54.2% vs 10.7%) and remission rates (30.5% vs 6.7%) with BTA.