Attention-deficit/hyperactivity disorder (ADHD) is one of the most common pediatric psychiatric disorders, occurring in approximately 5% of children.1 The disorder persists into adulthood in about one-half of those who are affected in childhood.2 In adults and children, diagnosis continues to be based on the examiner’s subjective assessment. (Box 13-9 describes how ADHD presents a complicated, moving target for the diagnostician.)
Patients who have ADHD are rarely studied with imaging; there are no established imaging findings associated with an ADHD diagnosis. Over the past 20 years, however, significant research has shown that molecular alterations along the dopaminergic−frontostriatal pathways occur in association with the behavioral constellation of ADHD symptoms—suggesting a pathophysiologic mechanism for this disorder.
In this article, we describe molecular findings from nuclear medicine imaging in ADHD. We also summarize imaging evidence for dysfunction of the dopaminergic-frontostriatal neural circuits as central in the pathophysiology of ADHD, with special focus on the dopamine reuptake transporter (DaT). Box 210,11 reviews our key observations and looks at the future of imaging in the management of ADHD.
Dopaminergic theory of ADHD
The executive functions that are disordered in ADHD (impulse control, judgment, maintaining attention) are thought to be centered in the infraorbital, dorsolateral, and medial frontal lobes. Neurotransmitters that have been implicated in the pathophysiology of ADHD include norepinephrine12 and dopamine13; medications that selectively block reuptake of these neurotransmitters are used to treat ADHD.14,15 Only the dopamine system has been extensively evaluated with molecular imaging techniques.
Because methylphenidate, a potent selective dopamine reuptake inhibitor, has been shown to reduce disordered executive functional behaviors in ADHD, considerable imaging research has focused on the dopaminergic neural circuits in the frontostriatal regions of the brain. The dopaminergic theory of ADHD is based on the hypothesis that alterations in the density or function of these circuits are responsible for behaviors that constitute ADHD.
Despite decades of efforts to delineate the underlying pathophysiology and neurochemistry of ADHD, no single unifying theory accounts for all imaging findings in all patients. This might be in part because of imprecision inherent in psychiatric diagnoses that are based on subjective observations. The behavioral criteria for ADHD can manifest in several disorders. For example, anxiety-related symptoms seen in posttraumatic stress disorder, social anxiety disorder, and panic disorder also present as behaviors similar to those in ADHD diagnostic criteria.
Molecular imaging might provide a window into the underlying pathophysiology of ADHD and, by identifying objective findings, (1) allow for patient stratification based on underlying physiologic subtypes, (2) refine diagnostic criteria, and (3) predict treatment response.
Nuclear medicine findings
In general, nuclear medicine investigations of ADHD can be divided into studies of changes in regional cerebral blood flow (rCBF) or glucose metabolism (rCGM) and those that have assessed the concentration of synaptic structures, using highly specific radiolabeled ligands. Both kinds of studies provide limited anatomic resolution, unless co-registered with MRI or CT scans and either single photon emission computed tomography (SPECT) or positron emission tomography (PET).
Synaptic imaging using radiolabeled ligands with high biologic specificity for synaptic structures has high molecular resolution—that is, radiolabeled ligands used for selective imaging of the dopamine transporter or receptor do not identify serotonin transporters or receptors, and vice versa. (Details of SPECT and PET techniques are beyond the scope of this article but can be found in standard nuclear medicine textbooks.)
SPECT and PET of rCBF
Early investigations of rCBF in ADHD were performed using inhaled radioactive xenon-133 gas.16 Later, rCBF was assessed using fat-soluble radiolabeled ligands that rapidly distribute in the brain in proportion to blood flow by crossing the blood−brain barrier. Labeled with radioactive 99m-technetium, these ligands cross rapidly into brain cells after IV injection. Once intracellular, covalent bonds within the ligands cleave into 2 charged particles that do not easily recross the cell membrane. There is little redistribution of tracer after initial uptake.
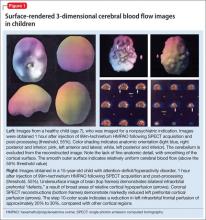
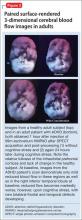
The imaging data set that results can be reconstructed as (1) surface images, on which defects indicate areas of reduced rCBF, or (2) tomographic slices on which color scales indicate relative rCBF values (Figure 1). Because of the minimal redistribution of the tracer, SPECT images obtained 1 or 2 hours after injection provide a snapshot of rCBF at the time tracer is injected. Patients can be injected under various conditions, such as at rest with eyes and ears open in a dimly lit, quiet room, and then under cognitive stress (Figure 2), such as performing a computer-based attention and impulse control task, or during stimulant treatment.
Numerous investigators have found reduced frontal or striatal rCBF, or both, in patients with ADHD, unilaterally on the right17 or left,18,19 or bilaterally.20 Additionally, with stimulant therapy, normalization of striatal and frontal rCBF has been demonstrated14,19—changes that correlate with resolution of behavioral symptoms of ADHD with stimulant treatment.21