Expert Commentary

Webcast: Factors that contribute to overall contraceptive efficacy and risks
In this webcast Dr. Burkman compares perfect and typical use failure rates of the 2 most popular forms of contraception, OCs and condoms, with...
Dr. Creinin is Professor and Director of Family Planning, Department of Obstetrics and Gynecology, University of California, Davis, Sacramento.
Dr. Schimmoeller is a Fellow, Family Planning, Department of Obstetrics and Gynecology, University of California, Davis, Sacramento.
Dr. Creinin reports receiving grant or research support (all of which goes to the Department of Obstetrics and Gynecology, University of California, Davis) from ContraMed, Medicines360, Merck & Co., and the National Institutes of Health/Eunice Kennedy Schriver National Institute of Child Health and Human Development. He reports being a consultant to Allergan, Danco, Estetra, Femasys, HRA Pharma, Lemonaid Health, Medicines360, and Merck & Co. and is a speaker for Allergan and Merck & Co.
Dr. Schimmoeller reports no financial relationships relevant to this article.

What this evidence means for practiceThis study provides further reassurance regarding the low risk of pelvic infection among women with an LNG IUS. Insertion of an IUS should not be delayed to await results of Chlamydia or gonorrhea testing in a woman without clinical evidence of pelvic infection. Risk-based, as opposed to universal testing, is imperative.12 These recommendations are in agreement with current recommendations of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists.13,14 Practices that employ 2-visit protocols unnecessarily limit women’s access to the IUS, as research has shown that nearly half of women desiring an IUD do not return for device placement if a second encounter is required.15
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.

In this webcast Dr. Burkman compares perfect and typical use failure rates of the 2 most popular forms of contraception, OCs and condoms, with...
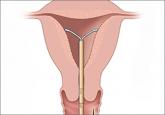
Although use of long-acting reversible contraception is increasing slowly in the United States, there is plenty of room for improvement,...

Yes. In 2008, the rate of unintended pregnancy was 54 per 1,000 among women and girls aged 15 to 44 years. In 2011, this rate dropped by 18%, to...

The US unintended pregnancy rate has reached an all-time high. Two experts explore the roots of the problem (cost most paramount) and offer...
