Potassium
Both DKA and HHS patients have total body potassium deficits due to osmotic diuresis that require careful repletion. Deficits can be substantial: The average total whole body potassium deficit in DKA is 3 to 5 mEq/kg.2 Clinicians should exercise caution, however, since DKA patients may be hyperkalemic initially despite a total body potassium deficit. Early hyperkalemia is due to the transmembrane shift of potassium secondary to acidosis and insulin deficiency as well as hypertonicity. If initial potassium is greater than 5.2 mEq/L, potassium should not be administered but instead rechecked in 1 to 2 hours. If the potassium level is 4.0 to 5.2 mEq/L, then 10 mEq per hour is usually adequate. For levels between 3.3 and 4.0 mEq/L, administer potassium chloride at 20 mEq per hour. For levels less than 3.3 mEq/L, insulin should be held and potassium chloride should be aggressively repleted at 20 to 30 mEq per hour with continuous cardiac monitoring.1-3 Failure to recognize and act on critical potassium levels is a known cause of unexpected death in DKA. During the first hour of DKA onset, patients are more likely to die from hyperkalemia. Later, while the patient is “stabilizing” on an insulin infusion, potassium levels will fall as insulin drives potassium back into cells.
Bicarbonate
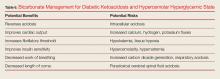
Bicarbonate has many theoretical benefits but also has potential risks (Table 6).
In general, bicarbonate should not be given to patients whose pH is above 6.9. Bicarbonate may provide some benefits to patients who have a pH below 6.9, but only in certain situations. Clinicians must always keep in mind that IV bicarbonate can cause a paradoxical respiratory acidosis in the central nervous system.2 When administered, bicarbonate increases serum bicarbonate which allows the pH to rise as the acidosis decreases, in turn decreasing the need for hyperventilation, with a resultant rise in carbon dioxide levels. Because carbon dioxidecan freely diffuse across the blood brain barrier but bicarbonate cannot, carbon dioxide levels rise in the cerebral spinal fluid (CSF), but there are no acute changes in CSF bicarbonate values. The result is an increased CSF carbon dioxide and an unchanged CSF bicarbonate value, resulting in an acute CSF respiratory acidosis. Additionally, bicarbonate has a very high osmolarity and is very hypernatremic relative to sera. Because of these potential deleterious effects of bicarbonate, we only recommend the rapid infusion of bicarbonate in DKA for hyperkalemic emergencies and impending cardiopulmonary arrest. Profoundly acidotic patients with pH values below 6.9 who are not improving with early aggressive care can potentially benefit from the careful administration of bicarbonate given relatively slowly.2 It is critically important that the EP recognizes that bicarbonate administration is the only therapeutic variable associated with cerebral edema in children with DKA and is usually seen within 4 to 12 hours after treatment.18Phosphate
Since there is no proven benefit to giving phosphate to adult patients with DKA, it is rarely used, except in specific situations other than pediatric DKA. Similar to potassium, initial serum phosphate levels do not reflect total body phosphate levels due to transmembrane shifts.2,3 Phosphate repletionis most beneficial for patients who have cachexia, respiratory depression, anemia, cardiac dysfunction, or phosphate values lower than 1.0 to 1.5. If given, 20 to 30 mEq/L potassium phosphate (K2PO4) added to fluids is usually sufficient.3 Overly aggressive phosphate administration (>4.5 mmol/h or 1.5 mL/h potassium phosphate) can cause severe hypocalcemia and should be avoided.1,3 In pediatric patients, up to one-half of potassium requirements are often given as potassium phosphate, but this may vary by institution.
Conclusion
Both DKA and HHS are diabetic emergencies that must be approached and managed systematically to correct underlying dehydration and metabolic abnormalities. Patient care begins by determining the etiology of these conditions, especially HHS. Once the cause has been identified, patients should be treated with bolus fluids to obtain adequate perfusion, followed by IV fluid infusion. Clinicians should carefully monitor the serum sodium level of patients with DKA or HHS to determine the ideal amount and type of fluid required, and also should measure potassium levels prior to starting patients on insulin. (Tables 7 and 8 summarize important clinical pearls when treating patients with DKA or HHS.)