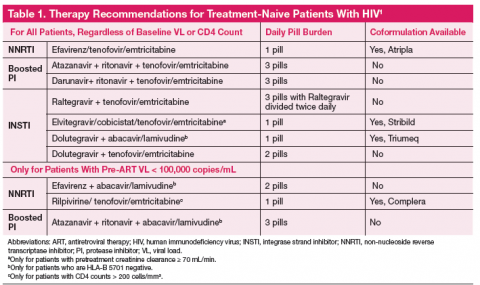
The one pill daily revolution in human immunodeficiency virus (HIV) management continues with the recent FDA approval of Triumeq, a combination tablet with an integrase inhibitor backbone containing dolutegravir, abacavir, and lamivudine. The first all-in-one protease inhibitor (PI) option is on the horizon and might receive approval before this article is published. The Department of Health and Human Services (DHHS) recommends 7 regimens for any treatment-naive patient with HIV, with 3 additional options if the patient has a baseline plasma RNA viral load (VL) < 100,000 copies/mL (Table 1). 1 With 10 first-line options and 4 once daily pill regimens, HIV treatment is poised to enter the realm of managed care.
Guidelines recommend treatment of all patients with HIV, both to slow disease progression, and to reduce the risk of HIV transmission. 1 Regimens should be individual ized based on vi rologi c efficacy, toxicity, pill burden, dosing frequency, drug interaction potential, drug resistance testing results, comorbid conditions, and cost. Therapy should only be deferred on a case-by-case basis. Patients considering therapy must be willing and able to commit to taking daily medications and understand the risks and benefits of therapy. Each antiretroviral (ARV) class, and each individual drug, has its own set of advantages and disadvantages. This article will review considerations when choosing a regimen, provide a brief overview of the first-line treatment options, and finally touch on cost considerations.
Choosing An Appropriate Regimen
Choosing an antiviral regimen has 2 basic steps. First, a provider must determine what choices are medically appropriate. An appropriate regimen has 3 active agents with acceptable performance and no contraindications. An ARV regimen generally consists of 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus 1 drug from 1 of the following classes: non-nucleoside reverse transcriptase inhibitor (NNRTI); PI boosted with ritonavir or cobicistat; or integrase strand transfer inhibitor (INSTI). The next step is helping the patient determine which regimen will suit him or her best considering dosing, food requirements, and potential adverse effects.
Genotype, Viral Load, and CD4 Testing at Baseline
A primary consideration is, “Will the regimen work?” This question can best be answered by the genotype, plasma RNA VL, and CD4 count. Baseline genotypes should be obtained for all patients and used to eliminate any inferior drug-resistant treatment options. Viral loads > 100,000 copies/mL eliminate 3 treatment options due to inferiority (inability to fully suppress the HIV virus) in patients with high VL (Table 1). Specifically, abacavir is not recommended (unless given with dolutegravir) with VL > 100,000 copies/mL. Rilpivirine is also not recommended for patients with VL > 100,000 copies/mL or CD4 < 200 cells/mm 3.
Creatinine Clearance
Many NRTIs require dosage adjustment when creatinine clearance (CrCl) falls below 50 mL/min. Cobicistat is a novel pharmacokinetic enhancer or boosting agent and is only recommended for baseline CrCl > 70 mL/min. 2
HLA-B 5701
If a patient has a positive HLA-B 5701 screening test, abacavir is strictly contraindicated due to the propensity for abacavir hypersensitivity, a potentially life-threatening reaction.
Allergies
Darunavir has a sulfonamide moiety and should be avoided in patients with a sulfa allergy.