Khandelwal and colleagues investigated the utility of a split-fill to decrease health care costs. In the splitfill process, patients were dispensed only days 1 to 16 of their oral anticancer medication at the initial fill. If the medication was tolerated and the prescribing provider deemed no changes in treatment necessary, the remaining 12 to 14 days of the cycle were then dispensed. Unfortunately, all insurance companies did not authorize the split-fill plan, thus preventing some patients in the study to participate in this cost savings strategy. However, it was determined for the patients who discontinued therapy, about 34% could have reduced wastage had they been on the split-fill plan, resulting in an average direct savings of $934.20 per patient who discontinued use. 9
In 2011, the Hematology/Oncology and Pharmacy divisions at the VA Pittsburgh Healthcare System (VAPHS) examined the issues surrounding dispensing and monitoring of oral anticancer therapy. Higher utilization of oral anticancer therapy was identified and in parallel, increasing rates of patient nonadherence, toxicity, and wasted medication. Originally, most providers dispensed oral anticancer therapy as a 1-month supply. However, in efforts to increase adherence, limit toxicity, and avoid medication waste, some oncologists began only dispensing a 1- to 2-week supply of medication per visit. This shift in practice led to a pilot study evaluating the utility of limiting all oral chemotherapy to a 7- to 14-day supply during the first 3 months of treatment.
Pilot Study
The goal of the pilot study was to increase adherence, decrease toxicity, and avoid medication waste. Patients who initiated a new oral anticancer therapy between August 15, 2011, and February 15, 2012, were enrolled in the pilot study. Each patient was to be provided only a 14-day supply of medication at each visit. Patients on concurrent chemoradiotherapy with capecitabine were dispensed only a 7-day supply (as they were at VAPHS daily for radiation) of medication. A pillbox designated for oral anticancer therapy was provided and filled by the clinical pharmacist before leaving the hematology/oncology clinic.
Patients were provided a calendar to record the time and date of their oral anticancer therapy selfadministration. Patients were also asked to record any missed doses and the reasons they missed taking the medication. In addition, patients were counseled on the importance of medication adherence, food-drug and drug-drug interaction, proper storage and administration of medications, and when/who to notify if AEs occurred.
Patients were asked to return the pillboxes to the hematology/oncology clinic for the next refill and meet with the clinical pharmacist. A pill count was performed at each visit in addition to screening for toxicity. If a toxicity was identified, the prescribing provider was contacted for further orders. If no changes were needed, the remaining 14-day supply was dispensed to the patient at that time. Adherence and toxicity were documented in the electronic medical record (EMR) at each visit.
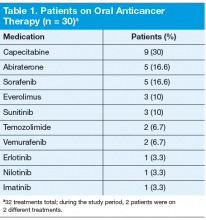
Thirty patients were started on 32 different oral anticancer therapies (Table 1) over the 6 months between August 15, 2011, and February 15, 2012. Patients already initiated on oral anticancer therapy before the start date were not included in this analysis. This number also did not include patients on lenalidomide, because this medication is mailed directly to the patient from a specialty pharmacy. All patients were male; average age was 68 (45-89) years; 83.3% of patients were white; and 83% of patients had a stage IV disease.