Lucas Franki is an associate editor for MDedge News, and has been with the company since 2014. He has a BA in English from Penn State University and is an Eagle Scout.
News
FDA approves mAb combo for hepatocellular carcinoma
- Author:
- Lucas Franki
Approval was based on results from the phase 3 IMbrave150 trial.
News
Aripiprazole lauroxil rivals paliperidone palmitate in schizophrenia
- Author:
- Lucas Franki
The baseline PANSS score was 94.1 for the aripiprazole patients and 94.6 for those who received paliperidone.
News

Hyperkalemia most common adverse event in women taking spironolactone
- Author:
- Lucas Franki
Of the 1,272 cases of hyperkalemia reported, only 25 occurred in women aged 45 years or less.
News

FDA approves Phexxi for use as an on-demand contraceptive
- Author:
- Lucas Franki
This is the first nonhormonal vaginal pH regulator contraceptive, the company says.
News
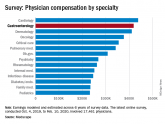
Before pandemic, gastroenterologist earnings were holding steady
- Author:
- Lucas Franki
Many gastroenterologists felt unfairly compensated in 2020, with only 52% reporting that they were satisfied with their salary.
News
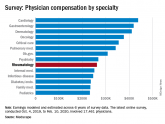
Before pandemic, rheumatologists saw small salary increase
- Author:
- Lucas Franki
Rheumatologists were more likely than the average physician to report that “having so many rules and regulations” was the most challenging part of...
News

FDA approves Fensolvi for central precocious puberty treatment
- Author:
- Lucas Franki
It is administered twice a year with a small injection volume to children aged 2 years and older.
News

FDA approves hyaluronic acid filler for lip augmentation, perioral rhytids
- Author:
- Lucas Franki
The manufacturer is “working to determine the appropriate launch timing and availability” of this new product.
News

FDA grants EUA to muscle stimulator to reduce mechanical ventilator usage
- Author:
- Lucas Franki
VentFree allows patients to begin treatment during the early stages of ventilation while they are sedated and to continue until the patient is...
News

FDA grants Breakthrough Therapy status to sotatercept for PAH treatment
- Author:
- Lucas Franki
In preclinical research, this investigational drug “reversed pulmonary vessel muscularization and improved indicators of right heart failure.”
News

FDA authorizes first COVID-19 test kit with home collection option
- Author:
- Lucas Franki
The Pixel test includes a specific Q-tip–style cotton swab for patients to use to collect their samples.
News
COPD, smoking independently associated with developing depression
- Author:
- Lucas Franki
The odds of developing depression in a year were 2.3%, 1.5%, and 1.4% in current, former, and never smokers without COPD, the investigators...
News

FDA approves Koselugo for pediatric neurofibromatosis treatment
- Author:
- Lucas Franki
Selumetinib for children who have NFI with symptomatic, inoperable plexiform neurofibromas is the first medicine...
News
SARS-CoV-2 escapes cotton, surgical masks of infected
- Author:
- Lucas Franki
All outer surfaces of masks used in the study were positive for SARS-CoV-2.
News

FDA removes pregnancy category C warning from certain MS medications
- Author:
- Lucas Franki
Labeling updates include more information on using certain MS drugs during pregnancy and breastfeeding.
