Take-Home Points
- Have a high-index of suspicion for MLLs and initiate treatment early.
- Compression needs to occur through short-stretch bandaging over a conventional Ace wrap in order to be successful.
- Apply the short-stretch compression with care to avoid shearing underlying tissue.
- Nonoperative treatment modalities require high patient compliance.
- MLLs need close monitoring until final healing occurs.
Morel-Lavallée lesions (MLLs) are traumatic degloving injuries resulting from separation of subcutaneous fat from underlying fascia. MLLs occur in association with acetabular fractures and are also associated with low-velocity crush injuries.1,2 Shearing creates a “false” space that is filled with hemorrhaged blood, fat, and lymphatic tissue.3 Disruption of the lymphatics leads to cavity formation and, eventually, a fibrotic pseudocapsule.4The pseudocapsule prevents resorption, leading to a chronic fluid collection, which potentiates the risk of infection or tissue necrosis.3,5,6 Skin necrosis may occur through direct-pressure compromise of the dermal vascular plexus.4 Necrotic skin may require multiple débridements, negative-pressure wound therapy or soft-tissue coverage, and may ultimately result in infection. MLLs classically occur in the greater trochanteric region, lateral thigh, buttocks, and back but also appear in the prepatellar region.1,3 Patients present with soft-tissue swelling, bruising, bulging, decreased cutaneous sensation over the region, and a palpable, fluctuant subcutaneous fluid collection with mobile skin.2,4,7 The mechanism of injury may cause a concomitant fracture. Magnetic resonance imaging (MRI), the preferred imaging modality, shows a discrete fluid collection between subcutaneous fat and underlying fascia. Ultrasonography may reveal a thickened capsule surrounding either a hypoechoic area or an anechoic area but its accuracy is user-dependent.7
Large MLLs may be treated with open serial débridement and healing by secondary intention; infection rates, however, are high. Authors have described several other treatment modalities, including percutaneous débridement with a brush followed by use of a large-bore drain and antibiotics; open débridement with meticulous dead-space closure; elastic compression bandaging; aspiration; and doxycycline sclerodesis.1,5,6,8,9 Modifications of short-stretch compression bandaging were recently described in edema control for hindfoot trauma, ankle trauma, and total ankle arthroplasty, but not for MLLs.10,11 Nickerson and colleagues4 retrospectively reviewed 87 MLLs, found that fluid aspirate of >50 mL predicted recurrence and failure with conservative measures, and recommended operative intervention for any MLL with >50 mL of fluid aspirated.
We report the case of an MLL that occurred in an unusual anatomical region, and we describe a novel application of a conservative treatment, which was selected on the basis of its success in lymphedema management. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
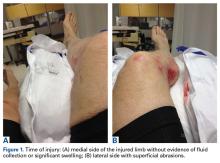
A 66-year-old man was injured when a parked vehicle began moving, pulled him under, and ran over his lower right leg. In the emergency department, no fractures or major injuries were noted (Figures 1A, 1B), and the patient was discharged.
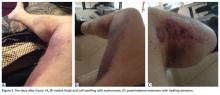
About 10 days after injury, profuse ecchymosis and swelling were noted running from the distal medial thigh to the proximal medial calf (Figures 2A-2C).
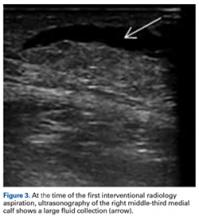
The leg had a palpable continuous fluid wave extending from the medial proximal thigh to the distal calf. The skin was hypermobile, and clinically an MLL was evident. Ultrasonography (Figure 3) and aspiration from interventional radiology 2 weeks after injury yielded 130 mL of blood-tinged fluid.Given the size of the MLL, the fluid collection reaccumulated. The patient was evaluated by an orthopedic traumatologist 3 days after the aspiration (17 days after injury).
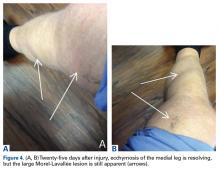
The treating physician suggested several options, including open débridement with cornrow stitching over a drain; minimally invasive débridement and irrigation over a drain; and compression. Nonoperative management was considered of limited success secondary to the size of the MLL. However, the patient selected nonoperative management with compression wrapping.Another orthopedic traumatologist confirmed the low likelihood that compression would resolve the MLL, given its size (Figures 4A, 4B).
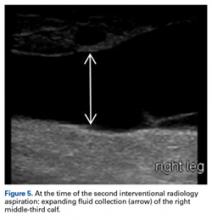
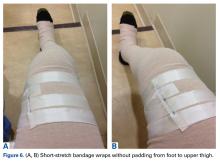
Another aspiration, 29 days after injury, yielded 235 mL of serous fluid (Figure 5). As expected, the lesion had reaccumulated. Compression wraps were continued for 2.5 weeks. The patient continued to have swelling in the foot, discomfort, and sleep disturbance as a result of bandage pressure. Maintaining the wrap’s position and pressure throughout the day was also proving difficult; he had to continually retighten the bandage.After the second orthopedic consultation, the patient saw a physical therapist trained in complete decongestive therapy. The therapist suggested placing short-stretch bandage wraps over the conventional long-stretch Ace bandage currently being used—a treatment common in lymphedema. The patient was wrapped from toe to groin without an initial layer of padding (Figures 6A, 6B), and the response was immediate.
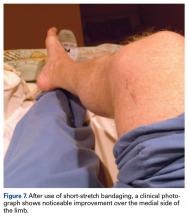
Given the improvement with short-stretch wraps, nonoperative treatment with monitoring was continued by the orthopedic surgeon.Nine weeks after injury, the leg was significantly improved, and clinical signs resolved (Figure 7).
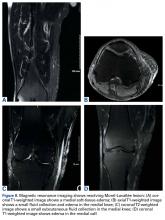
MRI with and without contrast showed no evidence of the MLL (Figures 8A-8D). Despite persistent discomfort, the swelling had subsided, and the traumatologist canceled the surgery. The patient was advised to start weaning off the wraps by going wrap-free a couple of hours each day. The trauma team was confident the infection risk was low, and the wraps were discontinued once the patient was pain-free, 7 months after injury.Discussion
Short-stretch bandaging has been performed mainly in lymphedema and ulcer management.
The bandage consists of woven cotton fibers that stretch to only 30% to 60% of the original length.12,13 By contrast, long-stretch bandages, such as conventional Ace wraps, are made of elastic fibers that stretch to 140% to 300%.12,13 Long-stretch bandages did not effectively control our patient’s swelling and had to be continually adjusted and retightened. Short-stretch bandages provide high working pressures and low resting pressures; Ace wraps work in opposite fashion.12-14 High working pressures occur from intermittent peaks in pressure with walking, creating a massage effect that results in reduced filtration of fluid from capillaries into surrounding tissue, promoting spontaneous contractions of lymphangions.13 These pressures decrease at rest and can improve patient comfort, especially at night.15 Although optimal pressures have yet to be determined, they have been estimated at 50 mm Hg to 60 mm Hg (supine) and 70 mm HG to 80 mm Hg (standing, with multilayer wrapping).13,15 Short-stretch bandages promote calf muscle pumping, provide edema containment, and improve peripheral venous and arterial flow.12 The efficacy of the bandages derives from the relationship of working and resting pressures, of containment and recoil.12 Used correctly, short-stretch bandages are applied in multiple layers and create an external force against calf muscle contraction, preventing the muscle from bulging outward as the bandages contract, thus forcing it to compress and pump the venous system.12 By contrast, long-stretch Ace wraps stretch with the muscle and, as edema increases, fail to provide adequate edema control.12 In addition, Ace wraps must be applied at a higher resting pressure to help effectively reduce venous reflux. Thus, patients experience continuous high pressures even when supine.14Compression bandaging reduces volume in lymphedematous limbs by reducing capillary filtration, shifting fluid into noncompressed parts of the body, increasing lymphatic reabsorption and lymphatic transport stimulation, improving venous pumping, and breaking down fibrosclerotic tissue.15 We think containment, improved venous flow, and enhanced muscle contraction contributed to the effectiveness of short-stretch bandaging as treatment for our patient’s MLL. Because MLLs also contain disrupted lymphatics, lymphedema management strategies (eg, short-stretch bandages) can be used. Our patient rapidly improved after conversion to short-stretch bandages.
These bandages are applied with 50% overlap to ensure even pressures throughout.16 Multiple layers are applied using a combination of spiral and figure-of-8 techniques, first clockwise and then counterclockwise, to avoid shearing underlying tissue.17 This method is very important in MLL treatment, given the degloving involved and the highly mobile skin and subcutaneous fat.
In standard lymphedema management, a foam padding layer is applied before the short-stretch bandage in order to reshape the limb and avoid proximal constrictions.13 In our patient’s case, the short-stretch wrap was applied without padding. Because his condition was acute, and the limb contour was preserved, limb reshaping and thus padding were not necessary.
Given the rapid, high-volume reduction that occurs within the first 1 to 2 weeks, bandages are reapplied daily to effectively adjust for the decreased swelling and altered limb shape.17 Most improvement is expected within the first few weeks—consistent with our patient’s case. Bandages usually are applied to the entire limb. For partial cases, the bandaging must extend past the area of swelling and incorporate the knee to prevent displacement of fluid into the joint.17 Feet and ankles are bandaged in dorsiflexion.17Several factors must be considered with short-stretch wraps. For example, pressure may need to be adjusted in patients with peripheral vascular disease. In patients with ankle-brachial indexes >0.5, it is safe to apply pressure up to 40 mm Hg.12 Reduced pressure is recommended for patients with arterial disease, sensory disturbance, lipoedema, poor mobility, frailty, or palliative needs.13The unusual location of our patient’s MLL accounts for the delay in diagnosis. To our knowledge, no other authors have reported such a large MLL in this location. A few series and case reports have listed MLLs in the calf near the gastrocnemius muscle, in the ankle, in the prepatellar area, and in the suprapatellar region, including the thigh,1,3,18-20 but there are no reports of MLLs running from medial thigh to proximal calf. MLLs of this size classically are treated surgically, but our patient selected nonoperative management.
To our knowledge, there are no earlier reports of using this nonoperative technique to treat MLLs. Conservative treatment with compression has been discussed, but no case involved short-stretch bandages. Large MLLs are thought to require surgery plus some type of drainage. The success of using short-stretch bandages in our patient’s case should prompt further investigation of use in adherent patients—which could ultimately result in reduced surgical needs, improved wound care (surgery is avoided), and a maintained low risk of infection. Although more work is needed to come to a more definitive verdict on this treatment method, it is a promising option that warrants consideration.
Am J Orthop. 2017;46(4):E213-E218. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.