Testosterone replacement works when indicated
When clinically indicated (ie, when a patient’s testosterone level is below 280 ng/dL and the patient is experiencing a variety of symptoms associated with hypogonadism), research has shown testosterone replacement therapy can improve sexual function, mood, and, in some cases, lean body mass and physical function.11,13
Keep in mind that the Endocrine Society and most professional organizations strongly discourage testosterone replacement in eugonadal men.3 Because of suppression of the HPG axis, men who discontinue testosterone replacement will typically experience symptoms of hypogonadism. Consequently, testosterone replacement should NOT be given to men with symptoms associated with hypogonadism (eg, fatigue or decreased libido) who do not have a low serum testosterone level.3
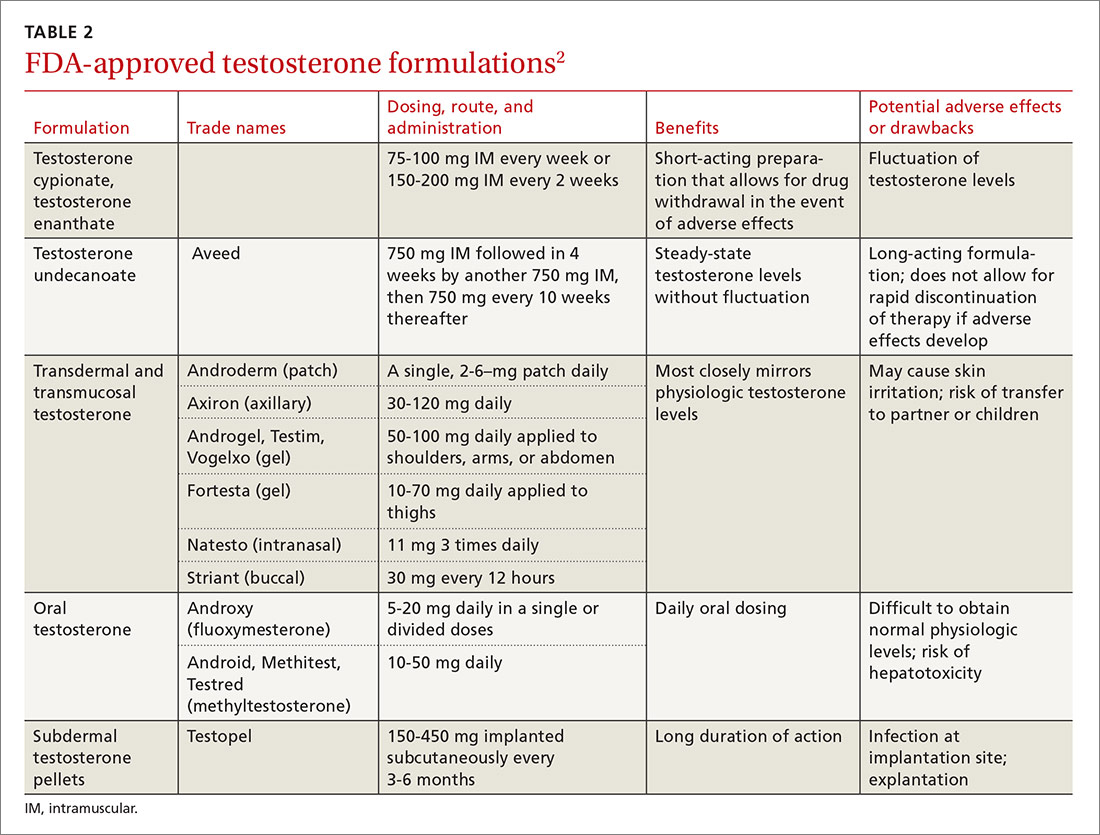
Testosterone is available in various forms, including oral, parenteral, pellets, transdermal gels and solutions, and as a buccal system. (Testosterone formulations and dosing information are described in TABLE 2.2) Oral formulations are generally not recommended due to potential hepatotoxicity and adverse effects on lipids.2 In addition, oral formulations have short half-lives, making it difficult to achieve and maintain normal testosterone levels.
Long-acting parenteral testosterone is effective but must be given as an intramuscular injection, usually at 2- to 4-week intervals. These preparations produce fluctuations in serum testosterone levels, with supranormal levels occurring soon after injection and subnormal levels occurring immediately prior to subsequent injections.14
Pellets that contain 75 mg of testosterone are implanted subcutaneously. The usual dose is 2 pellets (150 mg), but may be as high as 6 (450 mg). The dose can be titrated based on follow-up serum testosterone levels. The therapeutic effects of the pellets continue, on average, for 3 to 4 months, and up to as long as 6 months.
Transdermal testosterone preparations are the most commonly prescribed. These include gels, patches, and solutions. They are easy to use and achieve more stable serum levels that remain in a normal range with daily use.15
- Gels. Considerations when prescribing testosterone gel forms include the possibility of spread to female partners or children, leading to virilization and precocious puberty. The gel should be applied to the skin but not the genitals, and should be covered with clothing after drying for at least 5 to 10 minutes.
- Patches can be applied to the back, abdomen, or extremities. A skin rash occurs in about one-third of men who use testosterone patches and may lead to discontinuation.16
- Solutions are applied to each underarm daily. The starting dose is 60 mg under each arm; the dose can be adjusted based on follow-up serum testosterone levels.
- Buccal testosterone is applied to the buccal mucosa every 12 hours. It achieves therapeutic levels without large fluctuations. The tablet softens and forms to the gum, but does not dissolve and needs to be removed after 12 hours. The most common adverse effects are mucosal irritation and taste alteration.
Contraindications
Contraindications to testosterone replacement include heart failure, hepatic dysfunction (cirrhosis), prostate cancer, and breast cancer. Current guidelines also recommend not giving testosterone to men with severe lower urinary tract symptoms (due to benign prostate hyperplasia) with an International Prostate Symptom Score (IPSS) score >19.3 And, as mentioned earlier, the Endocrine Society strongly discourages testosterone replacement in eugonadal men.
After prescribing, monitoring is required
Men receiving testosterone replacement should have their testosterone levels checked at 3, 6, and 12 months after initiation of therapy, and annually thereafter.3 Therapy should be adjusted to achieve testosterone levels in the mid-normal range. Additional laboratory monitoring should include a serum hematocrit at baseline, at 6 months, and then annually if hematocrit remains in the normal range. Such testing is required because testosterone stimulates production of red blood cells from the bone marrow, which can lead to polycythemia. Discontinue therapy or reduce the dosage if a patient’s hematocrit rises above 54%, as there is a risk of thrombosis, although, in general, these events appear to be rare.3,8
Obtain a lipid panel, liver function tests. Lipid abnormalities—primarily a decrease in high-density lipoprotein (HDL) cholesterol—may occur with testosterone replacement. Obtain a lipid panel and liver function tests at baseline and then yearly during replacement therapy.
Keep an eye on PSA. Although testosterone replacement does not increase the risk of prostate cancer, the Endocrine Society still recommends obtaining a prostate specific antigen (PSA) level and performing a digital rectal exam in men 40 years of age and older prior to initiating testosterone therapy.
Do not prescribe testosterone replacement if the patient’s PSA level is >4 ng/mL (or >3 ng/mL in high-risk groups) or if there is a palpable nodule or significant prostatic hypertrophy. Repeat the PSA in 6 months and then annually as long as testosterone therapy is continued. Further evaluation for prostate cancer is warranted if the PSA increases more than 0.4 ng/dL/year.3,17